β-amino acid residue naming¶
General nomenclature¶
Acyclic β-amino acids come in four varieties, according to the substitution site (see the figure)
- β2
- Monosubstituted β-amino acid, the side-chain is on the α-carbon.
- β3
- Monosubstituted β-amino acid, the side-chain is on the β-carbon.
- β2,3
- Disubstituted β-amino acid, one side-chain on both the α- and the β-carbon.
- β-Alanine
- A simple β-backbone, without side-chains
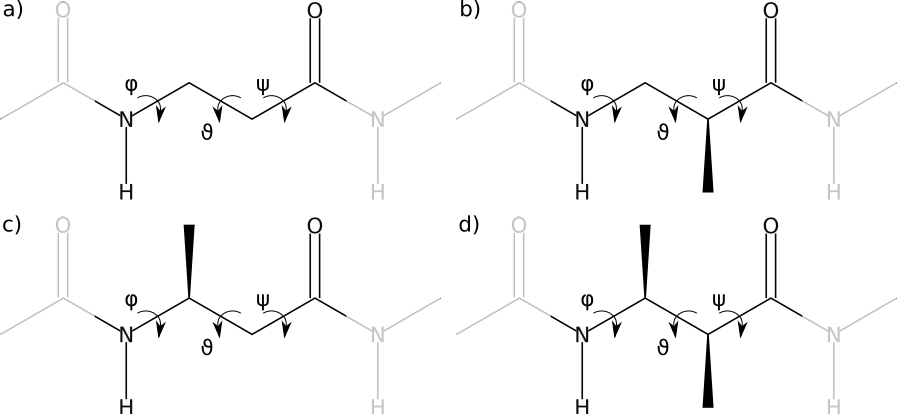
The four kinds of β-amino acids: bare β-backbone or β-alanine (a), β2-amino acid (b), β3-amino acid (c) and β2,3-amino acid (d)
In contrast to their natural counterparts, β-amino acids do not have a single, generally accepted nomenclature. We adopt here the following, widely used convention, which emphasizes the homology with α-amino acids and allows to account for the absolute conformation (chirality) as well. The general block format is :
<chirality><substitution type>h<side-chain designation>, where:
- <chirality>
- is the designation of the chirality of the substitution site atoms. For monosubstituted β-amino acids, this is either (S) or (R), including parentheses. For disubstituted ones, the substitution site is also labelled to avoid ambiguity, i.e. (2S, 3R) etc.
- <substitution type>:
- either β2, β3or β2,3.
- <side-chain designation>:
- single-letter abbreviation of the proteinogenic amino-acid whose side-chain is referred to. As for the chirality above, in the case of disubstituted amino-acids the substitution site must be explicitly given in order to avoid confusion, i.e. (2A,3Q), etc.
We include α-amino acids in this notation, too, with the following scheme:
<chirality>α<side-chain designation>, where:
- <chirality>
- is similar as for β2- or β3-amino acids, i.e. either (S) or (R). Additionally, for D and L are also supported for convenience [1].
- <side-chain designation>:
- is once again the single-letter abbreviation of proteinogenic amino acids
- Examples:
- monosubstituted:
- (S)β2hV: valine side-chain on the α-carbon with S chirality
- (R)β3hR: arginine side-chain on the β-carbon with R chirality
- disubstituted:
- (2S,3R)β2,3 h(2A,3L): disubstituted β-amino acid with an alanine side-chain on the α-carbon (with S chirality) and an arginine on the β-carbon (R chirality)
- achiral (bare backbone):
- βA
- α-amino acids:
- (S)αV: L-valine
- (R)αW: D-tryptophan
Two, more complicated examples are shown in the next two figures:
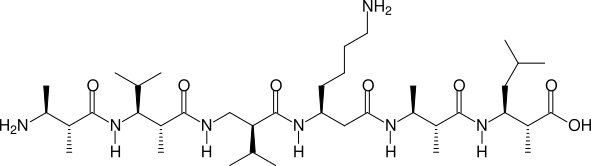
(2R,3S)β2,3h(2A,3A) - (2R,3S)β2,3h(2A,3V) - (S)β2hV - (S)β3hK - (2R,3S)β2,3h(2A,3A) - (2R,3S)β2,3h(2A,3L)
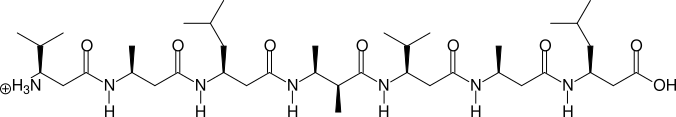
(S)β3hV - (S)β3hA - (S)β3hL - (2S,3S)β2,3h(2A,3A) - (S)β3hV - (S)β3hA - (S)β3hL
Simplified nomenclature¶
In the betafab2 command, essentially the above defined notation is used for defining a β-peptide sequence, with the following simplification:
- superscripts are omitted
- instead of the greek letter β the capital “B” is used
- commas in amino-acid abbreviations are omitted, they are used instead for separating the subsequent residues in the peptide chain
- letters S and R, describing the absolute conformation, are not italicized
For example, to construct the above two peptides, the following PyMOL commands can be used:
betafab2 hp6, (2R3S)B23h(2A3A), (2R3S)B23h(2A3V), (S)B2hV, (S)B3hK, (2R3S)B23h(2A3A), (2R3S)B23h(2A3L)
betafab2 valxval, (S)B3hV, (S)B3hA, (S)B3hL, (2S3S)B23h(2A3A), (S)B3hV, (S)B3hA, (S)B3hL
In addition, α-amino acids are also supported with the following notation:
betafab valval, (S)AV, (S)AA, (L)AL, (D)AV, (S)AA, (S)AL
betafab mixed, (S)AV, (S)B3hV, (R)B2hA, (S)AQ, (2R3S)B23h(2C3W)
Additionally, we now support the two most common cyclic beta-residues: 2-aminocyclopentanecarboxylic acid (ACPC) and 2-aminocyclohexanecarboxylic acid (ACHC) with the syntax:
betafab transachc, (2S3R)ACHC
betafab cisacpc, (2R3S)ACPC
Capping groups can also be added:
- N-terminal:
- ACE (acetyl)
- BUT (butyryl)
- C-terminal:
- NME (N-methylamide)
betafab ace_valxal_nme, ACE, (S)B3hV, (S)B3hA, (S)B3hL, (2S3S)B23h(2A3A), (S)B3hV, (S)B3hA, (S)B3hL, NME
Secondary structure¶
In addition to designating the amino-acids, the desired fold can also be given in the above notation. Each residue can be followed by a secondary structure descriptor in one of the following forms:
- A square bracket-enclosed, space-separated triplet (pair) of floating point numbers, e.g. (S)AQ[-57 -47] or (S)B3hA[-140.3 66.5 -136.8]
- The name of an entry in the secondary structure database, enclosed in curly braces, e.g. (S)AQ{Alpha-helix} or (S)B3hA{H14M}
| [1] | Note that because for β-amino acids no internationally agreed convention exists on the D / L nomenclature, their chirality can only be specified using the unambiguous S / R notation. |